ORIGINAL ARTICLE | https://doi.org/10.5005/jp-journals-10083-0935 |
Comparative Radiographic Study of Hydroxyapatite/Beta-tricalcium Phosphate with Bioabsorbable Membrane in the Management of Mandibular Class II Furcation Defects
1,5Mano Dental Clinic and Implant Centre, Chennai, Tamil Nadu, India
2Department of Periodontics, Tamilnadu Government Dental College, Chennai, Tamil Nadu, India
3Department of Dental Surgery, Government Theni Medical College and Hospital, Tamil Nadu, India
4Karthick Dental Clinic, Madurai, Tamil Nadu, India
Corresponding Author: A Selvam, Department of Dental Surgery, Government Theni Medical College and Hospital, Theni, Tamil Nadu, India, Phone: +91 9488058065, e-mail: drselvamperio@gmail.com
How to cite this article Manohar JJ, Kshirsagar JT, Selvam A, Mahalakshmi A, Maria TN. Comparative Radiographic Study of Hydroxyapatite/Beta-tricalcium Phosphate with Bioabsorbable Membrane in the Management of Mandibular Class II Furcation Defects. J Sci Dent 2021;11(1):2–7.
Source of support: Nil
Conflict of interest: None
ABSTRACT
Background: Management of moderate to advanced furcation defects presents a major challenge in periodontal therapy. Conventional treatments like root resection have led to limited success which led to attempts of regenerative therapy which can result in bone fill and closure of defects.
Aim and objective: To compare the outcomes of hydroxyapatite (HA)/beta-tricalcium phosphate (BTCP) with bioabsorbable collagen membrane (test group) to open flap debridement (OFD) (control) in the management of human mandibular class II furcation defects radiographically.
Materials and methods: A total of 20 furcation involvement sites (10 tests and 10 controls) were treated in this randomized clinical trial. Test groups were treated with OSSIFI (HA/BTCP) and HEALIGUIDE (collagen membrane). Control groups were treated with OFD alone. Defect depth, defect fill, and defect fill percentage were measured radiographically. p value <0.05 was considered significant.
Results: After 6 months, radiographic measurements showed a significant defect fill of 1.69 ± 0.55 and 1.18 ± 0.32 in test and control groups, respectively. The test group showed statistically significant improvements.
Conclusion: The combined use of HA/BTCP with collagen membrane demonstrated clinically better results and statistically significant improvement in defect fill in intraoral periapical radiograph (IOPA) compared with open debridement.
Keywords: Beta-tricalcium phosphate, Bioabsorbable membrane, Bony defects, Furcation, Furcation involvement, Guided tissue regeneration, Hydroxyapatite..
INTRODUCTION
Inflammation within the supporting tissues of the teeth, progressive attachment, and bone loss characterized by pocket formation and/or recession is called periodontitis.1 In such a scenario, tooth mortality is reduced in the majority of patients following periodontal treatment.2 In contrast, a higher frequency of tooth loss was observed in multirooted teeth with furcation involvement at the time of initial treatment.3 In periodontal therapy, the management of moderate to advanced furcation defects was always a challenge for dentists. In class II furcation involvement,4 teeth are associated with an increased risk of progressive loss of connective tissue attachment and alveolar bone resorption. Anatomical inaccessibility of the furcation lesions is the major factor for reduced success rate for therapy since it interferes with patients’ daily hygiene efforts as well as successful instrumentation during treatment.5 To overcome this issue, the surgical opening of the site before debridement can gain improved access to the furcation area.
Conventional treatments like root resection or “tunnelization” have led to limited success which led to attempts at regenerative therapy aiming at closure and bone augmentation of the defect area. As a result, various bone graft materials, such as, autogenous grafts, allogenous grafts, xenografts, and alloplasts, are being used.6,7 On periodontal applications, autogenous and allograft graft materials are of limited use on a routine basis due to inadequacy in donor material and the fear of the remote chance of disease transmission which led way for the development of alloplasts or synthetic bone substitutes for periodontal applications. The concept known as guided tissue regeneration (GTR)8 is based on the concept in which the epithelium is excluded from the periodontal defect and selective repopulation of the defect with the periodontal ligament, cementum, and bone is allowed.9
In this study, intraoral periapical radiograph (IOPA) evaluation of the regenerating potential in the treatment of class II furcation defects of mandibular molars using hydroxyapatite (HA)/beta-tricalcium phosphate (BTCP) with collagen membrane was aimed and done. IOPA evaluation opted as it was the most cost-effective and also less radiation exposure to patients when compared with other radiographic techniques.
MATERIALS AND METHODS
After obtaining institutional ethical committee approval, 20 subjects were randomly selected from the Department of Periodontics, Tamilnadu Government Dental College and Hospital, Chennai, India. Systemically healthy subjects around 20–45 years of age of either gender with furcation defect involving a minimum of 3 mm horizontal probing depth and gingival margin to be present at coronal to or at the level of the roof of the furcation after the completion of the initial phase of therapy were included. Subjects with poor oral hygiene maintenance after initial therapy, previous history of medication for past 6 months before the study, tobacco or tobacco-related products, pregnant/lactating women, known systemic diseases/metabolic disorders, any known allergies, history of previous periodontal surgical treatment, furcation at third molars and untreated non-vital teeth were excluded.
The 20 subjects were divided into two groups. Group I was treated with HA/ß-tcp (OSSIFI) and collagen membrane (HEALIGUIDE) and group II was treated only with open flap debridement (OFD).
All the subjects had undergone phase I therapy after complete intraoral evaluation and periodontal examination using a mouth mirror and Williams and Nabers periodontal probe under artificial light along with radiographic evaluation using IOPA.
After the re-evaluation of phase I therapy, the study sites were selected in the subjects and randomly allocated for groups I and II.
Surgical Procedure
In aseptic condition, under local anesthesia crevicular incision was made using the BP blade No. 15 on the facial and lingual surfaces along with the extension of an additional tooth on either side of the defected tooth. The furcation defect was exposed to an elevation of the full thickness mucoperiosteal flap using the periosteal elevator. Area-specific Gracey curette was used and thorough surgical debridement of soft and hard tissue was done along with copious 0.9% normal saline irrigation.
After surgical debridement, furcation defects of group I subjects were filled with Ossifi mixed with saline and covered with the HEALIGUIDE membrane. Open flap surgical debridement alone was done for group II subjects.
Using 3-0 braided black silk sutures, the mucoperiosteal flaps of all the subjects were repositioned and covered with periodontal dressing (Coe-pac™). Postoperative routine systemic antibiotics and analgesics were prescribed for a week for all the subjects (amoxicillin 500 mg, metronidazole 400 mg, ibuprofen 400 mg).
Radiographic Measurements
Intraoral periapical radiographs of the study sites were done at baseline, 3 months, and 6 months using long cone paralleling technique and XCP holders. Using corelDRAW software version x6, radiograph was analyzed after digitizing with a digital camera10 (canon powershot sx230 hs).11
Using the criteria set by Bjorn et al.12 and by Schei et al.,13 the reference points, such as, CEJ, BD, and furcation fornix (FX) were used for IOPA evaluation. CEJ is the cementoenamel junction of the subject site. In case of absence of CEJ due to restoration, the apical margin of the restoration was used as CEJ reference, BD is the most apical position of the intrabony defect (subject site). In pre- and postoperative IOPA evaluation, the distance between CEJ to BD and CEJ to furcation fornix (CEJ to FX)14 was calculated and its difference was assessed. The difference between them was considered as the depth of the furcation defect [depth of furcation defect = (CEJ to BD) – (CEJ to FX)]. The distortion between the radiographs (correlation factor) of each subject was estimated by measuring the distance between the cementoenamel junction and furcation fornix (anatomically non-variable distance). The correction factor was calculated as follows:

RESULTS
Defect Depth
Intragroup Comparison
A statistically significant difference in defect depth was found within group I on a comparison between the baseline to 3 months and 6 months. It revealed a statistically significant difference of 1.21 (p = 0.001) and 1.76 (p = 0.001). A statistically significant difference of 0.55 was found in the comparison between 3 months and 6 months (p = 0.008) (Figs 1 to 3 and Table 1).
A statistically significant difference in defect depth was found within group II on the comparison between the baseline to 3 months and 6 months. It was calculated as 0.60 (p = 0.01) at 3 months and 1.11 (p = 0.001). A statistically significant difference of 0.51 was found in the comparison between 3 months and 6 months (p = 0.002) (Figs 4 to 7 and Table 1).
Intergroup Comparison
Baseline comparison analysis revealed no significant differences between defects for groups I and II.
No statistically significant difference was found at 3 months [0.24 (p = 0.40)] and 6 months 0.28. At 3 months, the mean difference in defect depth between groups I and II was [0.24 (p = 0.35)] (Table 1).
Defect Fill
Intragroup Comparison
A statistically significant increase in bone fill at the defect was found for groups I and II at the end of 3 and 6 months. For group I, it was 1.15 ± 0.59 and 1.69 ± 0.55, respectively. For group II, it was 0.51 ± 0.37 and 1.18 ± 0.32, respectively (Table 1).
On comparing between 3 and 6 months, a statistically significant difference of 0.54 (p = 0.015) was found for group I and 0.67 for group II (p = 0.002) (Fig. 8 and Table 1).
Intergroup Comparison
At 3 months, the mean difference in defects between groups I and II was 0.64 which was statistically significant (p = 0.01). At 6 months, the mean difference in defects between groups I and II was 0.51 which was statistically significant (p = 0.02) (Fig. 8 and Table 1).

Fig. 1: Group I: baseline IOPA X-ray

Fig. 2: Group I: 3 months postoperative X-ray

Fig. 3: Group I: 6 months postoperative X-ray

Fig. 4: Group II: baseline IOPA X-ray

Fig. 5: Group II: 3 months postoperative X-ray
Defect Fill Percentage
Intragroup Comparison
Within groups I and II, a statistically significant increase in the mean defect fill percentage at 3 and 6 months on comparing with its baseline. Comparison between 3 and 6 months showed a significant increase of 20.11 (p = 0.016) for group I and 24.73 (p = 0.001) for group II (Fig. 9 and Table 1).
Intergroup Comparison
On intergroup comparison, a statistically significant increase of defect fill percentage of 17.67 (p = 0.029) was seen in 3 months and at 6 months the increase was not significant [13.07 (p = 0.06)] (Fig. 9 and Table 1).
DISCUSSION
As the inorganic content of bone is primarily of calcium HA which is the subset of calcium phosphate, it has led to the interest in the use of calcium phosphate groups for bone filling procedures. Hence, the efficacy of biphasic calcium phosphate with a bioabsorbable membrane in the management of furcation defects was opted due to its alloplastic nature and patient morbidity was not increased.
Ossifi is synthetically composed of HA and BTCP (purest form) in a ratio of 70/30. Its chemical nature attributes the osseointegration property and HA acts as an amphoteric ion exchanger. The negative ion of the HA surface leads to selective deposition of calcium and phosphate ions on its surface which stimulates new bone and apatite formation.
Oral hygiene, occlusal forces,15 smoking, pulpal status,16 root divergence, and root trunk length are the major factors that affect the outcome of furcation treatment and in turn, might impact regeneration therapy.
Fig. 6: Group II: 6 months postoperative X-ray
The use of barriers began with non-absorbable membranes but it had a major disadvantage due to its need for a second surgery for the removal of the barrier. This was overcome with the use of absorbable membranes in the second generation. It enhanced the tissue coverage, reduced the risk of loss of regenerated attachment, prevent microbial invasion, and reduced barrier exposure. As stated by Haney et al.,17 the critical factors influencing the success of GTR are clot stabilization, space provision, wound stability, epithelial cell exclusion, and complete gingival coverage.
The success of regeneration is primarily due to the adherence of the developing clot to the root surface for proper wound maturation and thereby regeneration occurs.18 It has given way to the development of collagen absorbable membrane since it is mainly present in periodontal connective tissue and comprises approximately 30% of the human body and functions as the extracellular framework. It also has an added advantage due to its hemostatic nature, thereby it enhanced the platelet aggregation and facilitated wound maturation by improving the initial blood clot and fibrin linkage formation.
The collagen membrane acted as a lattice for the periodontal ligament fibroblasts as it is infiltrated by vascular channels. In vitro studies proved the chemotactic nature of collagen for the fibroblast which in turn increased migration of cells to the space between the root surface and the collagen membrane. Clinical trials and animal studies showed that it is a weak immunogen and effectively inhibited the epithelial migration and promoted new connective tissue attachment by most of the collagen membranes.19
In this study, all patients except one responded favorably with the treatment procedures. Barrier membrane exposure was found in one patient and 1 mm marginal gingival recession was found in three patients.
On comparing the baseline to 6 months postoperative, a statistically significant improvement was found in both the groups. Group I showed defect fill of 1.15 mm at 3 months and 1.69 at 6 months compared with group II which showed 0.51 mm at 3 months and 1.18 at 6 months. Group I which had HA + β-tcp/HG showed significant defect fill than OFD patients. OFD patients showed significant bone fill compared with baseline which coincided with Meffert et al. which also showed a significant bone fill of 0.70 mm with OFD.20
| Group I | Group II | Group I vs group II | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| At base line | At 3 months | At 6 months | p value | At base line | At 3 months | At 6 months | p value | At 3 months | At 6 months | p value | ||
| Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | Mean diff | p value | Mean diff | ||||
| Defect depth | 3.04 ± 0.61 | 1.83 ± 0.64 | 1.28 ± 0.80 | 0.001* | 2.67 ± 0.53 | 2.07 ± 0.59 | 1.56 ± 0.43 | 0.001 | 0.24 | 0.4 | 0.28 | 0.35 |
| Defect fill | – | 1.15 ± 0.59 | 1.69 ± 0.55 | 0.015* | – | 0.51 ± 0.37 | 1.18 ± 0.32 | 0.002 | 0.64 | 0.01 | 0.51 | 0.02 |
| Defect fill (%) | – | 37.53 ± 18.59 | 57.6 ± 18.9 | 0.016* | – | 19.86 ± 14.30 | 44.59 ± 9.30 | 0.001 | 17.67 | 0.029 | 13.07 | 0.06 |
* Significant if p value ≤ 0.05

Fig. 7: Defect depth comparison
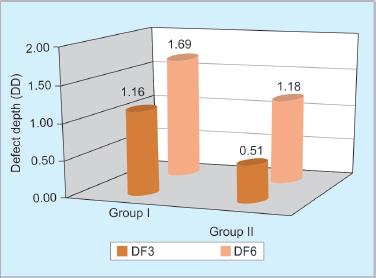
Fig. 8: Defect fill comparison
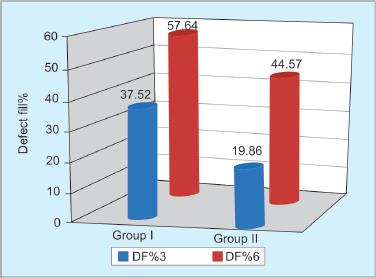
Fig. 9: Defect fill percentage comparison
Group I which had HA + β-tcp/HG showed a defect fill percentage of 37.53% at 3 months compared with 19.86% in OFD cases and 57.64% at 6 months compared with 44.59% in OFD cases. Though most of the defects treated either by HA + β-tcp/HG or by OFD resulted in considerable defect fill after 6 months, none of them showed complete filling of the furcation defects. This may be due to the limited period of the study.
Horwitz et al.21 stated that uses of radiographic parameters for evaluation of hard tissue changes in class II furcation defects are reliable. Hence, IOPA was used in this study for the evaluation of the depth of the furcation defect and defect fill to assess the treatment outcome. A statistically significant gain in-depth of the defect, the mean change in the area of defect due to the improvement in defect fill were observed in both the groups which coincided with the results reported by Pruthi et al.22 and Kenney et al.,23 respectively.
CONCLUSION
Regeneration is the ultimate aim of periodontal therapy, in our study, both groups showed a significant reduction in defect depth and increased defect fill in 6 months compared with their baseline values. The HA/BTCP + HG treated patients showed a significant reduction in defect depth and increased defect fill compared to the OFD patients.
CLINICAL SIGNIFICANCE
The most accurate method of accessing the hard tissue changes is an open bone assessment by surgical entry but due to its major drawbacks, such as, traumatizing the regenerated tissue, ethical concerns for surgical reentry, and more discomfort for the patient. To overcome these drawbacks, IOPA was utilized for evaluation as it was most cost-effective with minimum radiation exposure and minimal patient discomfort than other radiographic procedures.
The limitations of our study were the sample size, ethical concern for re-entry surgery for actual assessment of bone fill. Furthermore, long-term evaluation with well-controlled studies is required to confirm the actual outcome of this analysis.
REFERENCES
1. Garrett S. Periodontal regeneration around natural teeth. Ann Periodontol 1996;1(1):620–670. DOI: 10.1902/annals.1996.1.1.621.
2. Martin M, Gantes B, Garrett S, Egelberg J. Treatment of periodontal furcation defects. Review of the literature and description of a regenerative surgical technique. J Clin Periodontot 1988;15(4):227–231. DOI: 10.1111/j.1600-051X.1988.tb01575.x.
3. Hirsehfeld L, Wasserman BA. A long-term survey of tooth loss in 600 treated periodontal patients. J Periodontol 1978;49(10):495–512. DOI: 10.1902/jop.1978.49.10.495.
4. Glickman I. Clinical Periodontology.Philadelphia: Saunders; 1953.
5. Matia JI, Bissada NF, Maybury JE, Ricchetti P. Efficiency of scaling of the molar furcation area with and without surgical access. Int J Periodontics Restorative Dent 1986;6:25–35.
6. Mellonig JT, Bowers GM, Bright RW, Lawrence JJ. Clinical evaluation of freeze-dried bone allografts in periodontal osseous defects. J Periodontol 1976;47(3):125–131. DOI: 10.1902/jop.1976.47.3.125.
7. Sepe WW, Bowers GM, Lawrence JJ, Friedlaender GE, Koch RW. Critical evaluation of freeze-dried bone allografts iti periodontal osseous defects. Part II. J Periodontol 1978;49(1):9–14. DOI: 10.1902/jop.1978.49.1.9.
8. Gottlow J, Nyman S, Lindhe J, Karring T, Wennström J. New attachment formation in the human periodontium by guided tissue regeneration. Case reports. J Clin Periodontot 1986;13(6):604–616. DOI: 10.1111/j.1600-051X.1986.tb00854.x.
9. Wang HL, Boyapati L. “PASS” principles for predictable bone regeneration. Implant Dent 2006;15(1):8–17. DOI: 10.1097/01.id.0000204762.39826.0f J Periodontol 1971;42:685–690.
10. Prapayasatok S, Janhom A, Verochana K, Pramojanee S, et al. Digital camera resolution and proximal caries detection. J Dentomaxillofacial Radiology 2006;35(4):253–257. DOI: 10.1259/dmfr/32165678.
11. Scaf G, Sakakura CE, Kalil PFD. Comparison of simulated periodontal bone defect depth measured in digital radiographs in dedicated and non-dedicated software systems. Dentomaxillofac Radiol 2006;35(6):422–425. DOI: 10.1259/dmfr/61300663.
12. Bjorn H, Halling A, Thyberg H. Radiographic assessment of marginal bone loss. Odontol Revy 1969;20:165–179.
13. Schei O, Waerhaug J, Lovdal A, Arno A. Alveolar bone loss as related to oral hygiene and age. J Periodontol 1959;30(1):7–16. DOI: 10.1902/jop.1959.30.1.7.
14. Linares A, Cortellini P, Lang NP, Suvan J, Tonetti MS, On behalf of the European Research Group on Periodontology (ErgoPerio) Guided tissue regeneration/deproteinized bovine bone mineral or papilla preservation flaps alone for treatment of intrabony defects. II: radiographic predictors and outcomes. J Clin Periodontol 2006;33(5):351–358. DOI: 10.1111/j.1600-051X.2006.00911.x.
15. Rajendran M, Mahalakshmi A, Selvam A, Usha R. Interplay of occlusal forces and the periodontium. J Stomat Occ Med 2016;8(S1):17–24. DOI: 10.1007/s12548-016-0146-x.
16. Maheaswari R, Selvam A, Jeeva Rekha M, Mahalakshmi A, Jaishree TK. A guide to management of various endodontic periodontal lesions - a case series. Int J Cur Res Rev 2015;7(20):22–29.
17. Haney JM, Nilvéus RE, McMillan PJ, Wikesjö UM. Periodontal repair in dogs. Expanded polytetrafluoroethylene barrier membranes support wound stabilization and enhance bone regeneration. J Periodontol 1993;64(9):883–890. DOI: 10.1902/jop.1993.64.9.883.
18. Wikesjo UM, Nilveus RE, Selvig KA. Significance of early healing events on periodontal repair. A review. J Periodontol 1992;63(3):158–165. DOI: 10.1902/jop.1992.63.3.158.
19. Quteisch D, Dolby AE. The use of irradiated – crosslinked human collagen membrane in guided tissue regeneration. J Clin Periodontol 1992;19(7):476–484. DOI: 10.1111/j.1600-051X.1992.tb01160.x.
20. Meffert RM, Thomas JR, Hamilton KM, Brownstein CN. Hydroxylapatite as an alloplastic graft in the treatment of human periodontal osseous defects. J Periodontol 1985;56(2):63–73. DOI: 10.1902/jop.1985.56.2.63.
21. Horwitz J, Machtei EE, Reitmeir P, Holle R, Kim T-S, Eickholz P. Radiographic parameters as prognostic indicators for healing of class II furcation defects. J Clin Periodontol 2004;31(2):105–111. DOI: 10.1111/j.0303-6979.2004.00455.x.
22. Pruthi VK, Gelskey SC, Mirbod SM. Furcation therapy with bioabsorbable collagen membrane. A clinical trial. J Can Dent Assoc 2002;68:610–615.
23. Kenney EB, Lekovic V, Elbaz JJ, Kovacvic K, Carranza FA, Takei HH. The use of a porous hydroxylapatite implant in periodontal defects II. Treatment of class II furcation lesions in lower molars. J Periodontol 1988;59(2):67–72. DOI: 10.1902/jop.1988.59.2.67.
________________________
© The Author(s). 2021 Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by-nc/4.0/), which permits unrestricted use, distribution, and non-commercial reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.